
Biology 1
 |
Diffusion & Osmosis Lab |
 |
Background Information:
In the cells of plants and animals, cytoplasm is limited or
restrained from the environment by the existence of a membrane.
These membranes, by necessity, must be relatively
permeable to assist in such life processes as respiration and excretion.
Membranes must permit some substances to pass
through, yet prohibit other substances from doing so.
They may allow rapid diffusion of some substances or
very slow diffusion of others.
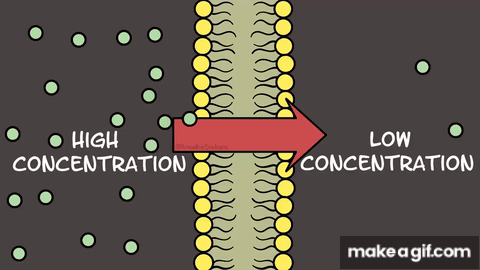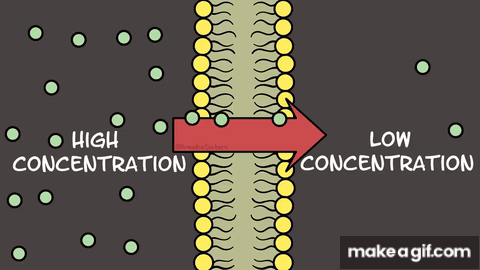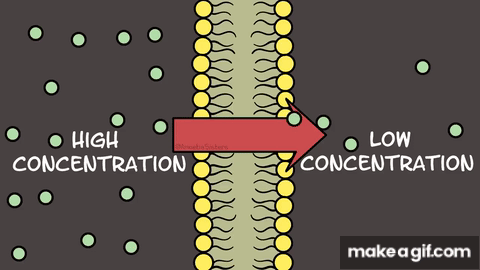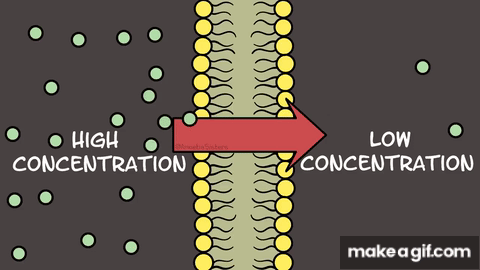
Diffusion is the movement of particles from areas of
high concentration to areas of low concentration.
Osmosis specifically refers to the diffusion of
water through a membrane.
Membranes can be referred to as differentially
permeable, selectively permeable, or semi-permeable.
In today’s lab, your group will perform an experiment which
will illustrate diffusion and osmosis.
You will be able to determine, through your
observations, that in a mixture of substances, some substances will diffuse
through a semi-permeable membrane and some will not.
NOTE:
This experiment will use several indicators.
(Indicator:
any substance that will detect the presence of other substances by turning a
different color.)
Lugol’s solution is a solution that contains iodine
which is an indicator for the presence of starch.
Iodine will turn a dark purple or almost black color
in the presence of starch.
The glucose test strips, obviously will detect the
presence of glucose.
The tips have been treated with a chemical that is
able to detect even the most minute particles of glucose in a substance.
A positive test for glucose will turn the yellow
testing area on the tip of the test strip a greenish color.
Since the testing area is really sensitive, you must
be very careful not to touch this with your fingers.
If you do, you may give a false positive test.
Today’s lab will try to answer this problem:
“Will glucose, starch, water, or iodine pass through a semi-permeable
membrane?”
1 plastic funnel
1 plastic cup
2 glucose test strips
Lugol's solution
pipette
stop watch (on cell phone)
liquid starch solution
glucose solution
electric pan balance
1. Fill the plastic cup with water to the top of the ridges on the cup as shown in the picture below.
2. To be sure that no contamination has occurred, test this water with a glucose test strip. There should be no glucose in the water at this point, so your test strip should be negative and should look like the negative test strip that is taped to the whiteboard in the front of the class. If your test strip does not look like this, dump the water out and get a new cup and start over.
3. Using the pipette, add 50 drops of the Lugol's solution to the clean water in the cup and swirl the cup around just a bit to mix the Lugol's solution with the water. The cup contents should look like a cup of "apple juice"....but don't drink it! Set this cup aside for later use in this lab. DO NOT CONTAMINATE THIS CUP WITH ANY OTHER CHEMICALS.
4. Obtain a dialysis tube membrane from Mr. Breitkreutz and prepare it as demonstrated at the beginning of class. Each group should work at their own sink area.
5. Tie a knot VERY TIGHTLY close to one end of the tube. Be careful not to rip the tubing.
6. Pour 2 cm (about the width of two fingers) of LIQUID STARCH solution into the tubing using the plastic funnel.
7. Add the same amount of GLUCOSE solution to the tubing following the same procedure as above for the liquid starch.
8. Tie another knot at the top of the tube to seal the contents of the tube.
9. Rinse the tube thoroughly under running water. Pay special attention to the top area of the tubing by the knot you just tied. Sometimes some residue is left in the flap that is at the very top. This must be rinsed thoroughly to remove any glucose or starch solution that may still be on the tubing.
10. Using the pan balance, find the mass of the tubing and record this number somewhere so that you can recall it after the experiment is over.
11. Carefully place the filled membrane into the cup of water/Lugol's solution that your prepared earlier. Set a timer on a cell phone for 10 minutes.
After 10 Minutes:
1. Observe the condition of the membrane tube. Making a mental note of its color change if any.
2. Use the other glucose test strip and test the water in the cup. Compare the results of this test strip to the test strips taped on the whiteboard at the front of the class. If the test strip is positive for glucose, hypothesize as to how the glucose go in the cup when you only put water and Lugol's solution in the cup at the beginning of the lab.
3. Use the pan balance to find the mass of the tubing. Compare this to the mass of the tubing at the beginning of the 10 minutes. Was the mass greater, was the mass less, or did the mass stay the same?
Clean-up Procedure:
1. Dump the water in the cup down the sink and rinse the sink out with running water.
2. Throw away the cup, tubing, and used glucose test strips in the garbage.
3. Use the blue spray bottle to clean the counter tops in your area near the sink
Your Assignment:
Complete the "Diffusion & Osmosis Lab Review" assignment that is posted on JupiterEd. Use the information on this lab as well as the powerpoint and video posted below.
|
|
|